Background and Aim: Iron deficiency anemia (IDA) is common in pregnancy and associated with both maternal and fetal morbidity. Despite its high prevalence and associated complications, there is a lack of consensus regarding the treatment of iron deficiency (ID) in pregnant individuals. In spite of the higher efficacy and tolerance of intravenous (IV) iron compared to oral formulations, obstetric guidelines reserve its use for IDA refractory to oral treatment, even in late gestation. This study sought to determine maternal and neonatal outcomes in individuals receiving IV iron supplementation antepartum, stratified by severity of IDA.
Methods: We performed a cohort study of pregnant individuals (age ≥18 years) who received at least one IV infusion of an iron formulation (iron sucrose (IS), low molecular weight iron dextran (LMWID), or ferric carboxymaltose (FCM)) during their pregnancy at a single center from 2011-2022. Our primary outcomes included both neonatal and maternal composite morbidity.Maternal composite morbidity was defined as postpartum hemorrhage, hysterectomy, puerperal infection, preeclampsia/gestational hypertension, maternal ICU admission, and/or requiring blood transfusion. Neonatal composite morbidity was defined as having either preterm birth, 5-minute Apgar less than seven, NICU admission, and/or low birth weight. Secondary outcomes included the incidence of pregnancy morbidity by anemia severity, defined by the World Health Organization Critiera as non-anemic (Hgb >11 g/dL, mild (Hgb 10 to 10.9 g/dL), moderate (Hgb 7 to 9.9 g/dL), or severe (Hgb < 7 g/dL), type of IV iron preparation used, and total iron dose received. Demographics, clinical characteristics, and perinatal outcomes were compared using chi-square or Fisher Exact test and two-sample t-tests.
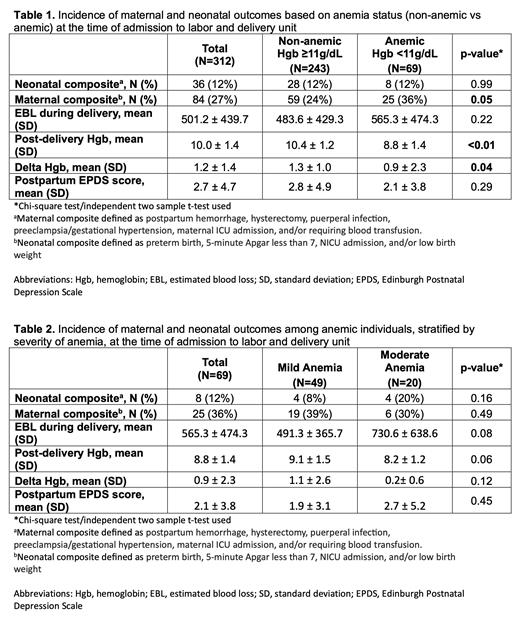
Results: A total of 312 pregnant individuals were included. The majority were 20-34 years (65.1%), multiparous (69.2%), and self-identified as being of White or Hispanic race (42.3% and 37.2%, respectively). Mean ferritin nadir during pregnancy was 12.9 ± 26.7 μg/L, which did not significantly differ between those with and without anemia. A total of 67.3% of individuals were anemic with Hgb < 11g/dL at some time during their pregnancy, with only 22.1% of individuals on admission to labor and delivery (L&D). In this cohort, most individuals (80.9%) received IV LMWID, 17.8% received IV IS, and only 1.0% received IV FCM. IV iron was well tolerated, with only 3 patients (0.96%) not completing their infusion due to reaction. More individuals with moderate anemia on admission received IV IS during pregnancy compared to those with mild and no anemia (50.0% vs 18.4% vs 15.0%, respectively, p<0.01). The mean number of infusions was 2.63±1.51 for IS compared to 1.20±0.40 for LMWID. Mean total iron dose received was 561±392 mg in those receiving IS compared to 979 ± 200 mg in those receiving LMWID. Only 23.4% of individuals received a total of 1,000mg or more of IS, compared to 91.3% of individuals who received LMWID. Anemia status was associated with the incidence of maternal composite morbidity, with more composite morbidity among anemic (25/69 (36.2%)) vs. non-anemic (59/243 (11.5%)) individuals (Table 1). In contrast, the incidence of neonatal composite morbidity did not significantly differ by anemia status. In those who were still anemic at the time of admission (N=69), the degree of anemia severity (mild vs moderate) was not significantly associated with maternal or neonatal composite morbidity (Table 2).
Conclusions: Anemia status, regardless of severity, is associated with higher rates of maternal composite morbidity. Anemia on admission to L&D was less likely in those who received IV LMWID compared to those who received IV IS. The total dose of iron received was lower, on average, in those prescribed IS (despite the need for more infusion visits). IV iron therapy was safe and effective in this cohort of pregnant individuals and decreased the incidence of anemia on admission to L&D.
Disclosures
Shatzel:Aronora Inc.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal